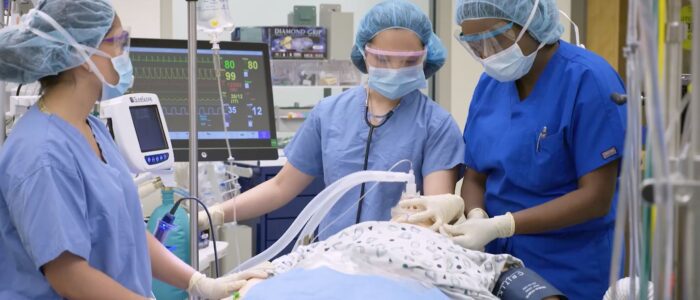
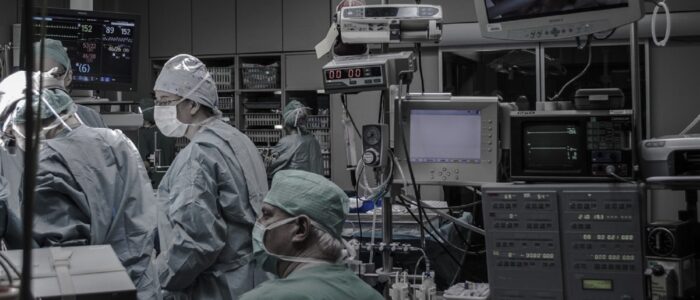
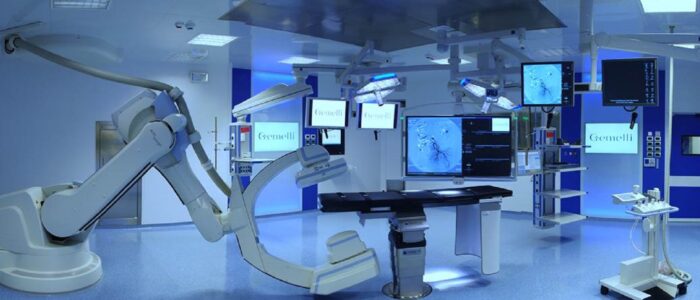
Most US states have marquee medical research and clinical delivery enterprises; most of them associated with one or more research universities. In many cases, these enterprises deliver the bulk of revenue to the university system; a topic we cover separately every month during our Healthcare teleconferences (See our CALENDAR).
Save for power and information and communication technology, the safety and sustainability requirements for university-affiliated healthcare systems are virtually identical to private, for-profit healthcare systems; even those for-profit systems that appropriate the word “university” in order to secure their brand. To be fair, most of them are “teaching hospitals”, though the medical profession (like most other professions) are always teaching. Conversely, many universities have close financial ties to for-profit healthcare systems. Students learn from off-campus clinical experience.
Both entities benefit from the possibility that cutting edge research is only footsteps away from the patient bed; and vice-versa — especially in cases where the university-affiliated hospital is the location for compassionate “right-to-try” treatment. University-affiliated hospitals have a statistical profile that should be understood in light of being the locus of last-resort treatment.
The AAMI bears the imprimateur of very well-financed non-profit organization; as one might expect for an organization servicing an industry that is about 25 percent of United States gross domestic product. The landing page for its standards catalog is linked below:
It is based in Arlington, Virginia, a city close to Washington D.C. that is home to many, many non-profit organizations. We maintain the AAMI catalog on the standing agenda of our Health colloquia. We also collaborate with the IEEE Education & Healthcare Facilities Committee and the IEEE Engineering in Medicine and Biology Society on a selection of healthcare electrotechnology issues related to medical instrumentation. See our CALENDAR for the next online meeting; open to everyone. All war stories and data — even anecdotal, messy data — are welcomed.
Issue: [Various]
Category: Academics, Healthcare Technology, Electrical, ICT
Colleagues: Mike Anthony, Robert G. Arno, Neal Dowling, Matt Dozier, Jim Harvey, Guiseppe Parise, Luigi Parise, Walt Vernon