Tag Archives: D1/1
- Home
- Posts tagged "D1/1" (Page 6)

The Kringle, Tulips & Tea
Good morning, internet friends that live on my phone.
☕️🤍☕️🤍☕️ pic.twitter.com/Dce7XnfpPb— Heather_in_WI (@Heather_in_WI) April 14, 2025
Bibliography:
ANS Z501 and ANS Z50.2:American National Standards for Baking Safety and Sanitation
US Food & Drug Administration: CFR Title 21 Part 136 Bakery Products
IEEE: Increasing energy efficiency of the gases production process in bakery ovens
Università del caffè
Illycaffè — commonly known as “Illy” — is an Italian coffee company sets high standards in the art and science of coffee culture. Founded by Francesco Illy in Trieste, Italy, in 1933, the company collaborates with artists to create innovative coffee-related products
Commemorative Coffee

Picture of the exterior of the Old Main Administration Building with students outside on the grounds before the fire. Click image for a view of the building after the fire.
Elon University Facilities Management
The Great Seattle Fire (1889) – University of Washington:
The University of Washington’s original campus was affected by the Great Seattle Fire in 1889. While not exclusively a college campus fire, it had a significant impact on the university.
University of Michigan Economics Building (1980):
Built in 1856. First chemical laboratory at a state university. Building served medical students and others as both laboratory and classroom. Situated just west and south of the original medical building. Additions made to the one-story building in 1861, 1866, 1868, 1874. In 1880 a two-story addition was added. In 1890, a three-story wing was added to the west of the original structure and was designed by E. W. Arnold of Detroit. A final addition was constructed in 1901. With the completion of the West Medical Building (later renamed the Dana Building) in 1903 and the Chemistry Building in 1909, the laboratories were transferred from the original Chemical Laboratory. In 1908, it became the Economics Building with Pharmacology occupying the north wing. Destroyed by an arson fire Christmas Eve 1981.
Seton Hall University Dormitory Fire (2000) – New Jersey:
A fire in a dormitory at Seton Hall University in 2000 resulted in three fatalities and numerous injuries. The incident led to increased awareness of fire safety in college campuses.
Harvard University Laboratory Fire (2006) – Massachusetts:
A chemical explosion and fire occurred at a Harvard University laboratory in 2006, resulting in injuries to several people. This incident highlighted the importance of safety measures in research facilities.
University of Missouri-Columbia Residence Hall Fire (2011) – Missouri:
A fire broke out in a residence hall at the University of Missouri-Columbia in 2011, leading to the evacuation of students. Fortunately, there were no reported injuries.
University of Delaware Chemistry Lab Fire (2013) – Delaware:
A laboratory fire occurred at the University of Delaware in 2013, prompting the evacuation of a chemistry building. No injuries were reported, but it emphasized the need for safety protocols in academic laboratories.
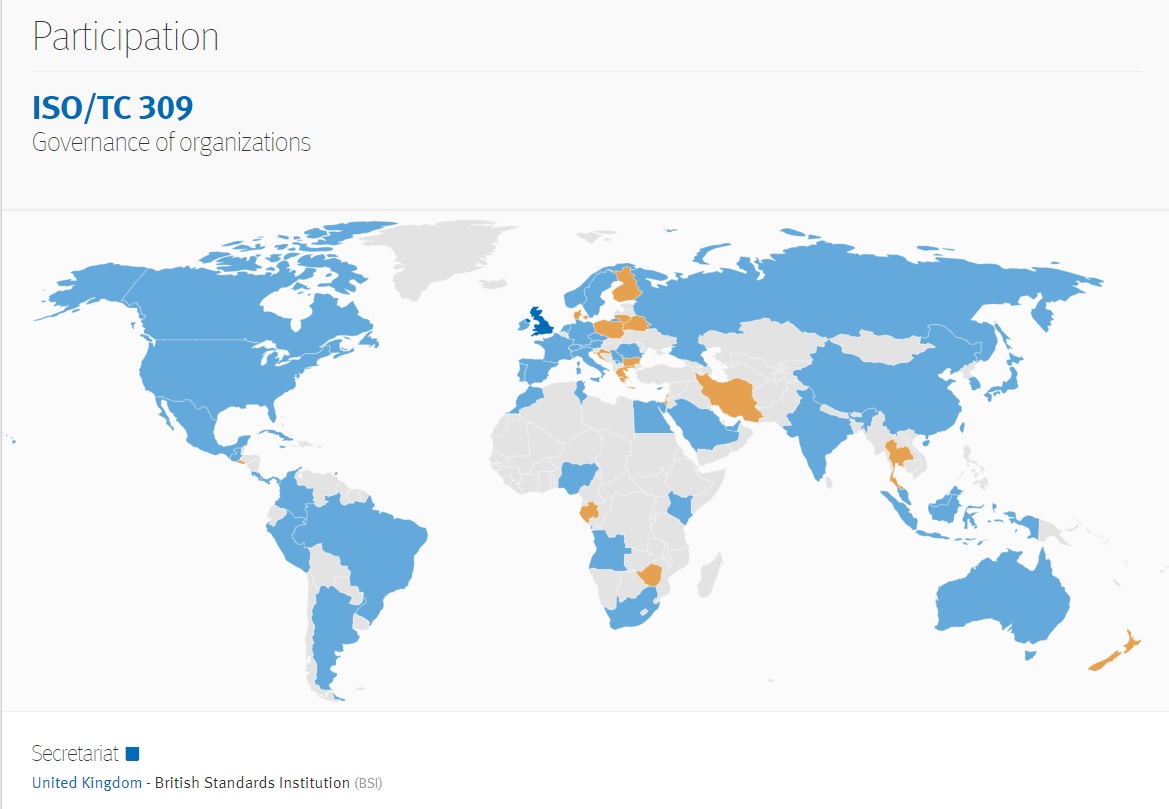
Governance & Accountability of Organizations
A relatively new International Standardization Organization committee is developing a consensus product that sets the broad contours of standardization in the field of governance relating to aspects of direction, control and accountability of organizations. The foremost aim of international standardization is to facilitate the exchange of goods and services through the elimination of technical barriers to trade. We find technical barriers to trade (TBT) a growing discussion in the education facility industry; especially in research universities that deal with highly specialized products in laboratories. We see TBT issues show up purchasing regulations for federally-sponsored projects hosted on federally-sponsored facilities.
The strategic business plan of ISO/TC 309 is linked below:
Executive Summary ISO Technical Committee 309 (ISO/TC 309)
The US organization charged by the American National Standards Institute with administering the US Technical Advisory Group (US TAG) is the InterNational Committee for Information Technology Standards (INCITS). The INCITS committee will operate under the ANSI-accredited procedures for US TAGS. Click here for more information about joining the US TAG or other INCITS committees. Jennifer Garner is listed as the contact person (jgarner@itic.org).
We do not find any public commenting opportunities on this project at the moment — January of every year tends to be a slow time in the standards world — but continue to monitor it through INCITS. We keep this committee’s work products on our International Standards monthly teleconference. See our CALENDAR for the next online meeting; open to everyone.
Issue: [18-157]
Category: Administration & Management, International, Finance, Academics, Public Policy
Colleagues: Mike Anthony, Christine Fischer
Thomas Hobbes was a political philosopher who advocated for **absolute sovereignty** as the only way to ensure peace and security in a commonwealth¹². He believed that people in their natural state were in a constant state of war, and that they needed to surrender their rights and freedoms to a strong ruler who could protect them from violence and chaos³⁴. Therefore, Hobbes wanted **larger** government control, as he viewed government primarily as a device for ensuring collective security¹. He justified this by arguing that people consented to the authority of the sovereign in exchange for their safety⁵..
Thomas Hobbes’s philosophy of government is relevant today because it addresses some of the fundamental questions and challenges that modern societies face, such as:
- How to justify and limit the authority of the state over its citizens?
- How to balance the rights and duties of individuals in a social contract?
- How to deal with the threats of violence, anarchy, and civil war?
- How to promote peace, security, and cooperation among nations?
Hobbes’s answers to these questions may not be the same as ours, but they can stimulate our thinking and help us evaluate our own assumptions and arguments. Hobbes’s Leviathan is widely regarded as one of the most influential works of political philosophy in the history of Western thought, and it has inspired many thinkers and movements across different disciplines and ideologies.
Leviathan 400
Thomas Hobbes titled his book “Leviathan” (1651) as a metaphorical representation of the powerful and all-encompassing state. In it, he proposed a social contract theory, arguing that individuals willingly surrender some freedoms to a sovereign authority in exchange for protection and order. The Leviathan, a biblical sea monster, symbolizes this sovereign entity’s immense power and control. Hobbes believed that without a strong central authority, human life would be chaotic and marked by a “war of all against all.”
The Leviathan concept underscores his advocacy for the largest possible administrative state to provide jobs that claim to maintain social stability and prevent anarchy. We cover the topic today because insofar as technology standards are concerned, federal involvement has run to excess (ignoring market signals), is just another form of market-making that ignores the limits placed upon us by Mother Nature.
Keep in mind that the American National Standards Institute administers a relatively small part of standards-setting in the United States. (Click here for a complete list; updated frequently. A great deal of ANSI resources are devoted to supporting members that run “conformance shops” essential to safe and fair trade. Other standards setting is done by governments, open-source, and other ad hoc consortia.
The culture of the standards-setting domain in every nation rests upon the culture of conformance. “Standards are the seed corn for conformance revenue” we are fond of saying and most of the expertise in the standards setting domain rests with people who make a living making sure others conform to the standard; whatever that standard may be. Conformance (product testing, installation certification, periodic re-commissioning and audits, etc.) is where the money is so we should not be surprised at the degree to which the user-interest (represented by the third gray column in our logo) is outnumbered.
That much said, having the global standards system is better than not having one at all. This system places a check on oligarchies that make central governments grow. The topic is relevant to our work because a surprising measure of influence over technical standards is undertaken by non-technical people who lack the sensitivity to technical and economic trade-offs — many of them presented by Mother Nature herself.
0. Leviathan.
Office of Management and Budget
United States Department of Education
United States Department of Commerce
United States House of Representative Committees
United States Senate Committees
Health, Education, Labor & Pensions
United States Constitution 10th Amendment
The foregoing block of content will be re-configured to another, more dynamic post that synchronizes with state and county-level standards action. Reminder: We do not advocate with governments at any level; merely follow it since the action of government appears routinely in the leading practice discovery activity of standards setting organizations.
IEEE-USA Government Policy Committees
Health 300
Today we break down regulations, codes, standards and open-source literature governing the safety and sustainability of university-affiliated medical research and healthcare delivery facilities. In large measure, the safety and sustainability agenda of the university-affiliated healthcare system infrastructure coincides with the private sector. Accordingly, we confine our interest to systems — water, power, telecommunication and security; for example — that are unique to campus-configured, city-within-city risk aggregations.
We usually start with a scan of the following titles:
International Building Code (with particular interest in Section 308 Institutional Group I)
K-TAG Matrix for Healthcare Facilities
NFPA 70 National Electrical Code Article 517
NFPA 99 Healthcare Facilities Code
NFPA 101 Life Safety Code Chapters 18 & 19
ASHRAE 170 Ventilation of Healthcare Facilities
Some of the content in the foregoing links need weekly refresh. We’ll get to that, time permitting.
Starting 2023 we break down our coverage of standards thus:
Health 200 Clinical delivery
Health 400 Research
We will thumb through the titles published by HL7 and NSF International — both Ann Arbor-based organizations. A surprising number of medical data companies are domiciled in Ann Arbor; not far from our own offices on State Street. We will also see if any bills and resolutions introduced into the 117th Congress will make into public law.
Finally, we collaborate with the IEEE E&H Committee on the following IEC committee projects from IEC/TC 62 Electrical equipment in medical practice.
– Common aspects of electrical equipment used in medical practice
– Diagnostic imaging equipment
– Equipment for radiotherapy, nuclear medicine and radiation dosimetry
– Electromedical equipment
![]()
As covered in previous posts, the original University of Michigan standards enterprise was one of the founding members of what has become ISO/TC 304 Healthcare organization management — following the lead set by Lee Webster at the University of Texas Medical Branch. Since last month’s colloquium ISO TC/304 there has been a fair measure of the usual back-and-forth that we will cover in today’s colloquium. We will examine the ideas in play in the links below today and try to organize them ahead of balloting:
Legacy Workspace (N.B. We are still in the process of uploading content onto the new University of Michigan Google Site facility)
Open to everyone. Use the login credentials at the upper right of our home page.













More
Health Insurance Portability and Accountability Act (HIPAA)
Health care cost as percentage of Gross Domestic Product for six representative nations.
Association of Academic Health Centers
International Conference on Harmonization: The ICH guidelines provide guidance on the development of pharmaceuticals and related substances, including clinical trials, drug safety, and efficacy.
Animal Welfare Act and the Institutional Animal Care and Use Committee
Good Laboratory Practice: GLP is a set of principles that ensure the quality and integrity of non-clinical laboratory studies. It ensures that data generated from non-clinical laboratory studies are reliable, valid, and accurate.
Visiting a newborn intensive care unit in Lviv hospital
New update alert! The 2022 update to the Trademark Assignment Dataset is now available online. Find 1.29 million trademark assignments, involving 2.28 million unique trademark properties issued by the USPTO between March 1952 and January 2023: https://t.co/njrDAbSpwB pic.twitter.com/GkAXrHoQ9T
— USPTO (@uspto) July 13, 2023
Standards Michigan Group, LLC
2723 South State Street | Suite 150
Ann Arbor, MI 48104 USA
888-746-3670